Reduce Internal Defects and Enhance Efficiency
Welcome to the ‘Internal Defects and Deviations’ module, a pivotal component of Wismatix QMS designed to help your organization minimize internal defects and deviations. Detecting and managing these issues are critical for optimizing your workflow and ensuring product and process quality. By effectively addressing the root causes of defects and deviations, you can not only reduce waste but also maximize profitability.
This module offers a comprehensive set of features, from seamless defect registration to in-depth case management and analysis. It empowers your team to detect, investigate, and prevent future occurrences, fostering a culture of continuous improvement and efficiency within your organization.
- Effortless Defect Registration: Easily record internal defects and deviations from PC, tablet, or mobile devices, promoting quick identification and resolution.
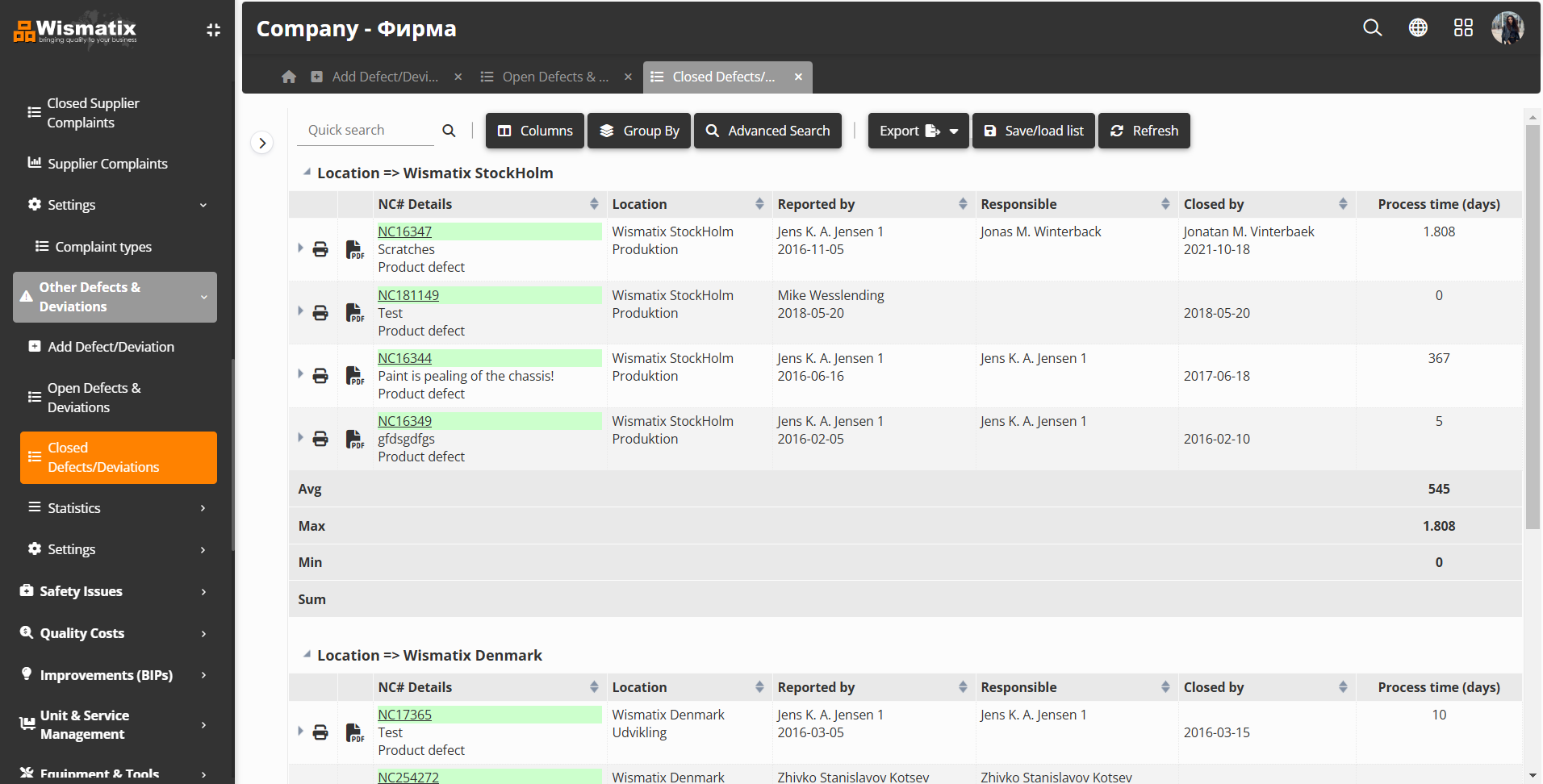
- Comprehensive Lists: Access organized lists of open and closed defect and deviation cases with advanced search, customization, grouping, and detailed search options. Save and export customized lists for future reference.
- Detailed Case Management: Each defect and deviation case includes essential details such as defect type, order numbers, problem description, immediate solutions, root-cause investigation, risk analysis for recurrence, preventive actions, images, documents, and the ability to create improvement proposals.
- Efficient Reporting: Generate comprehensive defect and deviation reports for informed decision-making and future prevention.
- Assigned Case Managers: Assign responsible case managers to ensure accountability and timely resolution.
- Processing Time Monitoring: Track case processing times, helping your team meet response time expectations and improve internal efficiency.
- Data-Driven Analysis: Utilize drill-down diagrams for in-depth analysis of internal defects and deviations, identifying trends and opportunities for process improvement.
- Effortless Defect Registration: Simplify defect registration, reducing time and effort while ensuring accuracy.
- Comprehensive Lists: Easily access and manage defect lists, enhancing organization and efficiency.
- Detailed Case Management: Quickly review and handle defect cases, facilitating effective resolution and continuous improvement.
- Efficient Reporting: Streamline reporting processes, enabling transparent communication and decision-making.
- Assigned Case Managers: Clarify responsibilities, leading to faster defect resolution and preventive actions.
- Processing Time Monitoring: Stay on top of response times, improving overall process efficiency.
- Data-Driven Analysis: Gain valuable insights through data analysis, supporting data-driven decisions and continuous improvement efforts.
- Reduced Internal Defects: Streamlined defect registration and resolution processes lead to a reduction in internal defects and deviations.
- Enhanced Efficiency: Efficient defect management results in improved workflow efficiency, reducing waste and maximizing profits.
- Improved Product Quality: Detailed case management and root-cause analysis enable your company to identify and address underlying issues, enhancing product quality.
- Transparent Communication: Quick report generation and communication facilitate transparency and informed decision-making.
- Optimized Processes: Clear assignment of case managers and monitoring of processing times lead to more efficient internal processes.
- Informed Decision-Making: Analyzing defect data through drill-down diagrams empowers your company to make informed decisions for continuous improvement.
Summary:
In summary, the ‘Internal Defects and Deviations’ module empowers your organization to reduce internal defects and deviations while optimizing efficiency and promoting a culture of continuous improvement. By effectively managing and preventing these issues, you can enhance product quality, reduce costs, and maintain a competitive advantage in your industry.